|
Last summer, I wrote about the experience of being interviewed by our CAHNRS (College of Ag + more) media writer, Seth Truscott. He's informed us that the news article ended as #7 across all WSU stories in the top research coverage of 2023.
0 Comments
Silas Bossert and Elizabeth Murray have an opening in the lab for a master's student project. This will involve lab work, field work, conservation, and pollinators. Please see the description in the pdf & email [email protected] with questions.
The Entomological Society of America's annual meeting was held in National Harbor, Maryland. Shout out to Hannah, 2nd year PhD student. She got 2nd place for her talk in the 10 Minute Student Paper Competition in the SysEB: Genomics session. Hannah's talk title was "Bee Genome Evolution: Uncovering Variability of Genomic Dark Matter". Is this great or what? We have a beautiful logo for our Bees of the World NSF grant.
New article out by Almeida, Bossert ... [more authors including the NSF Bees of the World gang, Danforth, Murray, Branstetter, and Freitas] ... & Pie. "The evolutionary history of bees in time and space", in Current Biology. Comprehensive study of bee phylogeny (using UCEs), age, and biogeography. What is pretty cool is that it serves as a comparative treatment to Charles Michener's classic 1979 paper on bee biogeography. Michener posited that bees originated in Western Gondwana, but it hasn't been adequately tested using molecular phylogenies and new methodologies. Another thing that I think is amazing -- the molecular dating. I'm talking 185 bee fossils used -- and not just node calibrations! Silas did the dating analysis, using a fossilized birth-death model (in the program MrBayes), meaning he assigned fossils to clades and not to a specific node or branch.
We had some media attention on this, too! Kind of interesting to see how that process worked. Our College of Ag, CAHNRS, put out an article and then WSU Insider published it a couple of days later. Several internet sites picked up the story put out by WSU, such as Popular Science. Silas and I did a radio interview for Northwest Newsradio. Silas was interviewed by NPR!
WSU distributes their news articles (like ours) on EurekAlert, so that other sites can access them for content. Some internet sites put their own 'creative' spin on it -- like the one that was titled "A team of paleontologists find fossils that could radically change what is known about bees" on Crast.net. Hm.... not exactly. This paper didn't deal with describing new fossils, but it was the most extensive use of fossil data on a bee molecular phylogeny. Silas Bossert has moved into a tenure track position at WSU. Congratulations! The UCE Phylogenomics Workshop for year 2 of our National Science Foundation grant (NSF-DEB 2127744) was held at the Bee Lab at the Utah State University campus in Logan, UT (it will be held at Washington State University in year 4). The workshop ran from June 4 – 11, 2023, with 12 participants and five instructors. It was designed to serve graduate students, post-docs, and/or faculty members seeking to enhance their knowledge and skills in developing phylogenomic datasets. The week was fun and info-packed! Participants were provided with comprehensive training and hands-on experience in the latest methodologies and techniques relevant to obtaining and analyzing ultraconserved elements. Through a combination of lectures, lab experiences, and practical tutorials and exercises, attendees gained an understanding of the UCE workflow – from DNA extraction and steps in library preparation, to the bioinformatic pipeline and preparing data for a comparative analysis in R. The instructors think that we were successful in having an environment of active learning, engagement, and knowledge exchange. Participants had the opportunity to meet scientific peers, allowing for valuable networking and collaboration opportunities. We surveyed the attendees on the first and last days to gauge impact of the workshop, and the feedback was positive. Participants reported an increase in skills and knowledge, and some of the highest impact areas were: 1) understanding of the Phyluce pipeline (used to process UCE data), 2) describing the purpose of a bead clean step, and 3) knowing the difference between a concatenated and a coalescent phylogenetic analysis. Stay tuned for 2025, when we host the UCE Phylogenomics Workshop in Pullman, WA! Also great news - there are NSF funds to help support travel and meals.
New Lab Member Alert! Lexi is joining us in the MS program. She is co-advised by Rich Zack (professor and curator in the MT James Entomological Collection). We are excited to welcome Lexi Menth to the lab. She is starting at WSU this summer so that she can get a jump on fieldwork. Lexi is undertaking a cool project -- an insect survey of the Fairchild Air Force Base, located west of Spokane. She'll be using various techniques to collect insects, such as light trapping, pitfall traps, and sweep netting. We are working with the US Fish and Wildlife Service, and Silas Bossert is also a PI on the grant. Everyone is anticipating the results of the next two years of sampling!
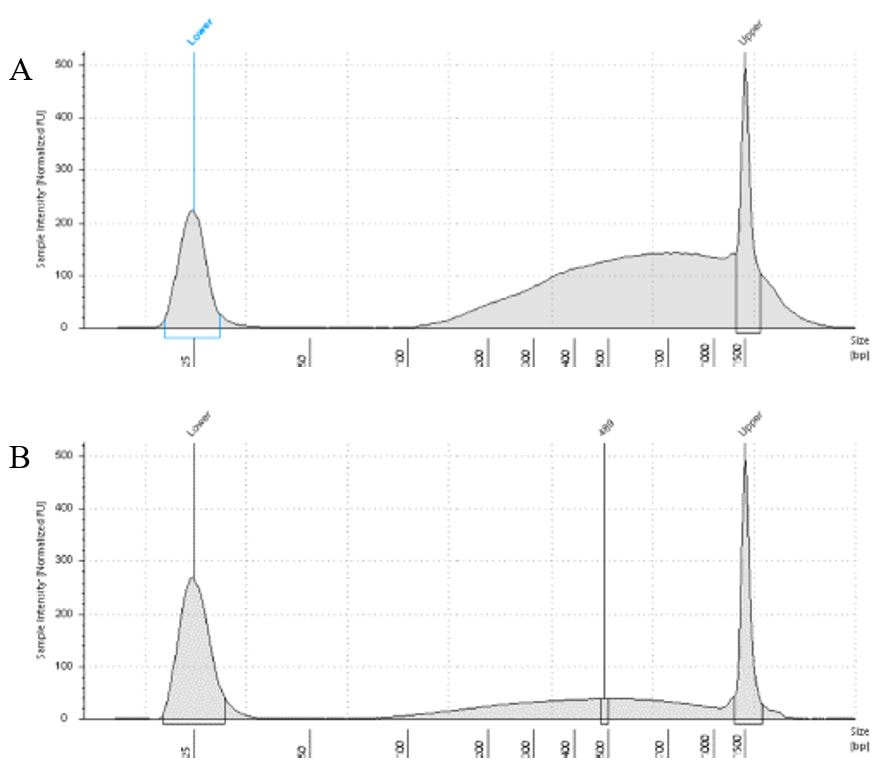
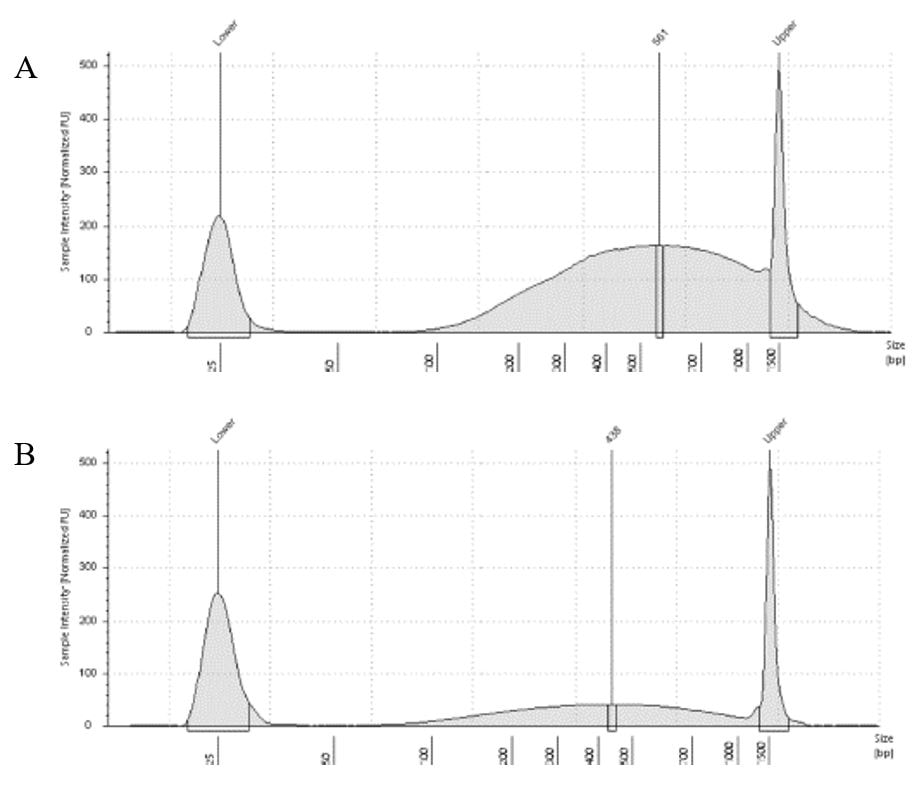
The lab has been getting the molecular protocols for next-gen sequencing up and running. One of the primary tools we have for our phylogenomics work is to do low-coverage genome sequencing on an Illumina sequencer. For the short-read sequencing, we actually NEED fragmented DNA (we're targeting 250-600 basepairs total to submit to sequencing (with adapters, etc.). We need to ascertain the quality of the DNA extract then make an educated guess as to how long to shear the DNA to get it broken into the ideal size pieces. We have a lot of old specimens with degraded DNA, and then we may choose not to shear at all. We're using a Bioruptor here at WSU. After getting our sheared sample, we go through a Kapa kit-based library preparation in the lab. Although I think it's stressful to purposely fragment the DNA, as you'll see below, it is critical that you really commit to it and often do a few cycles in order to obtain smaller pieces -- the long fragments will not be useful downstream anyway! Guest post by postdoc Felipe Freitas.Felipe interprets results from comparisons done in the lab with super-high quality DNA (intact, long strands) vs. some that is expected to be degraded. The first step of preparing libraries for Illumina sequencing is fragmenting the input DNA into a desired range of ~300-600 bp. Typically, there are two ways for doing that: mechanic fragmentation and enzymatic fragmentation. Mechanical fragmentation has been more widely used because of many factors, but mainly because its unbiased and can be more consistent about the fragment size obtained. Besides these factors, despite requiring a higher initial investment (buying the equipment, which is generally a sonicator), throughout time it turns cheaper because you don’t need anything else than tubes to accommodate your DNA, and electricity and water for the machine to work. We are starting to setup our lab for preparing libraries to sequence Low Coverage Genome using Illumina technology. And, once we have a sonicator (Dianogene Bioruptor plus) available at WSU we decided to perform some tests to establish the best parameters to fragment the DNA we will be using for our projects. Most of our projects rely on museum specimens, which have, generally, DNA degraded in some levels. After consulting the available manual of the equipment (https://www.diagenode.com/en/protocols/dna-shearing-for-standard-and-plus-protocol), and a blog post (https://igatechnology.com/blog/exome-seq-dealing-with-degraded-samples/) about the same topic and having the same questions – samples with some level of fragmentation, we prepared an experiment to certify that our setup would produce the results we are expecting considering the kind of samples we have. For our experiment we selected two samples: one with a less degraded or more contiguous DNA, extracted from a fresh specimen, and another one with degraded DNA, extracted from a museum sample. it was expected that these samples would give us two different scenarios or levels of fragmentation, so we would be able to evaluate how different configurations of the sonicator will influence the different samples. We then undertook a TapeStation analysis for these two samples to have a sense of the distribution of fragment sizes in both samples before the sonication. The results show that our expectations were right, the fresh specimen had DNA with fragment sizes mainly longer than we were able to read with the screen tape we had currently available (Figure 1.A), which can detect fragments between 25 and 1500 bp. Conversely, the museum sample had a fragment distribution ranging between 100-1500bp, with a peak around 620bp (Figure 1.B), which is slightly above the mean fragment size we were targeting. We then used two configurations of the sonicator to fragment the DNA: the first one had two cycles with the machine on for 30 seconds and off for 60 seconds. While the second one had three cycles with the same pattern 30/60. 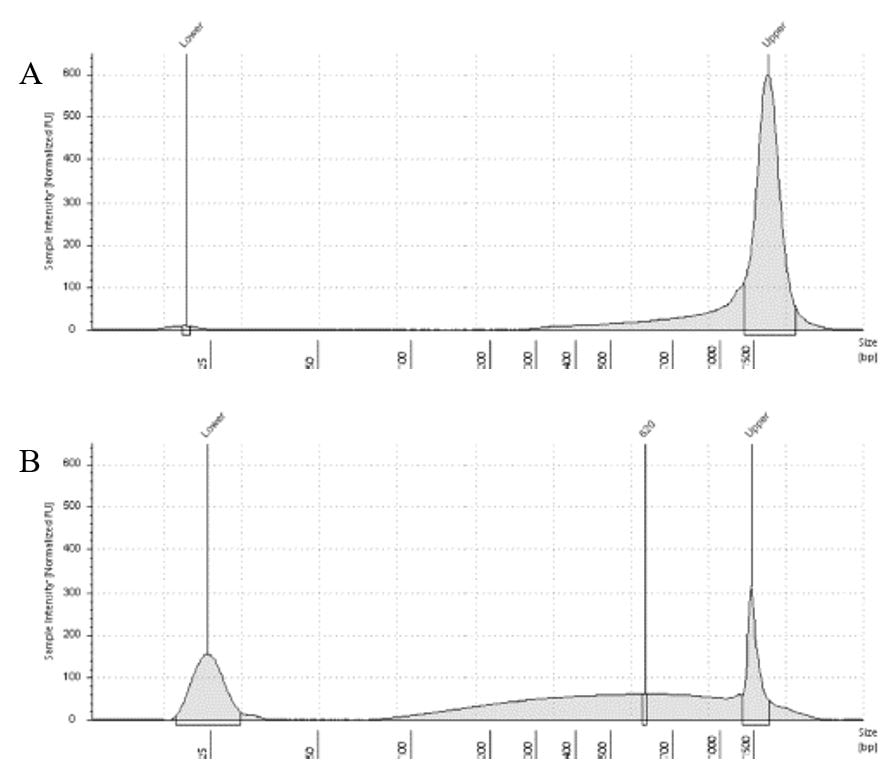 Figure 1: Fragment distribution on samples before sonication. A- More intact DNA. B- More degraded DNA. We had only high sensitivity tapes to use when conducting the experiment, that’s why we don’t see any peaks of fragments in A, but we infer that the peak is above the upper bound, considering that this is a fresh sample. The results of the first round, with two cycles, show that in fact the peak of concentration of our intact DNA was above the upper bound (Figure 2-A), because now we are able to see the peak around 700bp, which still above the fragment distribution we are targeting (300-600). However, for our degraded sample the distribution reached somehow the distribution and mean we were targeting. It was between 100 and 1000bp with the peak at 489bp.
The results of the second round, using three cycles, showed a different pattern. The more intact DNA (Figure 3-A) now reached the fragment size we were targeting, with a peak around 560bp. On the other hand, the more fragmented sample (Figure 3-B) showed a peak around 438b, only a slightly difference compared to the results of when it was exposed to two cycles. In conclusion, we can say, that based on the results of this simple test, for museum specimens, two or three cycles of 30/60 on the Dianogene Bioruptor sonicator are enough to reach the fragment distribution of 300-600 when the DNA are not too fragmented as in the case of our museum sample. While for samples with high quality DNA (mean >1500bp), at least three cycles would be necessary to reach the fragment distribution needed for preparing Illumina libraries. However, it is important to highlight that our sampling does not represent the variation of DNA degradation (or fragment distributions) that can be found in museum specimens. The level of DNA degradation will, generally, vary according to many factors, for example: how the specimen was collected if using cyanide, ethyl acetate or ethanol; if it was dried in an incubator or not; if it was stored in desired conditions of temperature and humidity; etc. A new year and a new lab member! We are excited to welcome Tatiana to the lab as a PhD student, co-advised by Elizabeth Murray and Silas Bossert. Tatiana Bush joined the lab as a PhD student and is diving into classes. She is supported from the collaborative NSF grant "Bees of the World -- Phylogenomics, Biogeography, and Evolution of Host-Plant Associations". Tatiana came to WSU from her undergrad and master's at the University of California, Riverside. She has experience in phylogenetic methods and host plant associations.
|
what's new in the lab?posts about the things we're doing Archives
January 2024
Categories |







 RSS Feed
RSS Feed
